Which of the Following Best Describes Exothermic Chemical Reactions
Which phrase best describes a solvent. The following chemical equation can be used to describe the reaction.

Gcse Science Daily Revision Task 43 Endo Exothermic Reactions Gcse Science Gcse Chemistry Science Flashcards
We can not tell anything about kinetic energy.

. 3 MULTIPLE CHOICE OPTIONS. B Photosynthesis is an exothermic reaction so more energy is absorbed breaking bonds than is released making bonds. In exothermic reaction heat is released from the system to the surroundings thereby temperature of the surroundings increases.
For the reaction A B 2 C which of the following best describes how we can measure the reaction rate. The reaction is endothermic. The same number of atoms but of different elements.
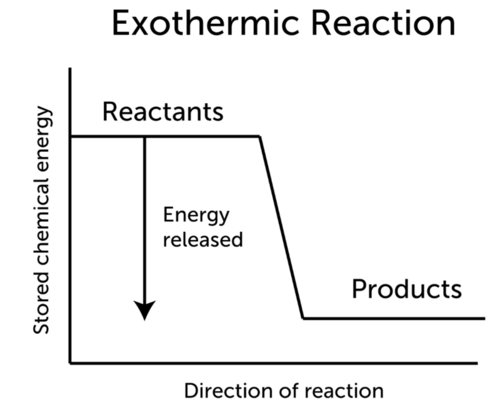
The energy of the products is lower than that of the reactants. Can be used to make medicine. So for the exothermic reactions enthalpy of the reaction must be negative ie ΔH is negative.
D Photosynthesis is an exothermic reaction so more energy is absorbed making bonds than is released breaking bonds. The chemical energy potential energy of reactant is higher than energy of product in exothermic reaction while chemical energy potential energy of reactant is less than energy of product in endothermic reaction. In thermodynamics the exothermic reaction describes a reaction which releases energy from the system to its surroundings usually in the form of heat.
The graph below represents a chemical reaction. Hope it will be helpful. The edited gene is more likely to mutate and cause disease.
Exothermic because energy is. Which of the following statements about chemical reactions are true. Exothermic reactions occur at a rapid rate.
A A chemical reaction occurs between the iceberg and the ocean. The products of endothermic reactions are lower in energy than the reactants. A process that requires energy to break the bonds and stores energy in the bonds which are formed in the reaction is an endothermic reaction.
So the correct option is C. Reactants Products Energy. C graphite H 2 O g CO 2 H 2g ΔH1314 kJ.
Fewer atoms of different elements. A 3 B 1 2 and 3 C 1 3 and 4. Either of the above procedures can be used.
So option a is correct. An exothermic reaction refers to a physical or chemical reaction that releases heat. Releases more energy than it absorbs.
The products of endothermic reactions are lower in energy than the reactants. D It affects nearby wildlife. We cannot determine which of.
The reaction is exothermic. An exothermic reaction is a reaction in which energy is released in the form of light or heat. Endothermic reactions store energy and exothermic reactions release energy.
Which of the following best describes exothermic chemical reactions. What is released by an exothermic. C graphite H 2 O g CO 2 H 2g ΔH1314 kJ.
Exothermic reactions give off heat. For example combustion is a. Endothermic because energy is absorbed.
An exothermic reaction occurs when energy is released during a chemical reaction often times when chemical bonds are made. The process in which energy is released when bonds are broken is a exothermic reaction. Thus in an exothermic reaction energy is transferred into the surroundings rather than taking energy from the surroundings as in an endothermic reaction.
The reaction has a high activation energy. Fats oils and cholesterols are all types of. The products of exothermic reactions are lower in energy than the reactants.
So option a is correct. An iceberg melting in an ocean is an example of an endothermic process because. Endothermic because energy is released.
Based only on this information which of the following is true. So potential energy of product is less than potential energy of reactant. In order to follow the conservation of matter the products of the chemical equation will have.
If an exothermic reaction is reversed it becomes an endothermic reaction. This reaction is best described as. In an exothermic reaction change in enthalpy ΔH will be negative.
In an energy diagram for an exothermic chemical reaction which of the following is true. A substance with a pH of 6 is called. Which phrase best describes an exothermic chemical reaction.
Which of the following is formed when an atom gains or looses an. C The iceberg absorbs thermal energy from the surrounding water. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products.
Genetically modified organisms can affect the environment around them. Endothermic reactions occur when electrons are shared between atoms but exothermic reactions occur when electrons are transferred between atoms Endothermic reactions involve changes in the nucleus of an atom but exothermic reactions do not involve changes in the nucleus. C Photosynthesis is an endothermic reaction so more energy is absorbed breaking bonds than is released making bonds.
B The iceberg releases thermal energy from the surrounding water. So for the exothermic reactions enthalpy of the reaction must be negative ie ΔH is negative. A They never occur spontaneously B They always occur spontaneously C They always release heat D They never release heat.
The energy was created during the reaction. Can be dissolved by the solute. Decomposition reactions are exothermic in nature so release heat.
For a chemical reaction the heat of reaction is equal to the A potential energy of the reactants B potential energy of the productsonly. Which of the following best describes exothermic chemical reactions. A chemical reaction that includes the release of energy in the form of heat or light is known as an exothermic reaction.
In endothermic reaction heat. In a particular chemical reaction 2 bonds are broken and no bonds are formed. Exothermic because energy is absorbed.
The reactants in a chemical equation have 3 carbon atoms 6 oxygen atoms and 4 hydrogen atoms. Can create organisms that survive in different environments.

Understanding Endothermic And Exothermic Reactions Chemistry Experiments Exothermic Reaction Chemical Reactions

Exothermic And Endothermic Processes Introduction To Chemistry

Exothermic Reactions Release Energy Endothermic Reactions Consume Energy Exothermic Reaction Homeschool Science Chemistry
Comments
Post a Comment